Introduction: We have recently shown that high frequency (2 x week) low dose (50 mg)(HFLD) rituximab (RTX) and acalabrutinib is highly effective initial therapy for patients (n=38) with progressive CLL (ClinicalTrials.gov NCT03788291, PMID:36689726). The first dose of 50 mg of IV rituximab administered at 25 mg/h decreased the median circulating CLL cell count by 84% from pre-treatment baseline at 1h with no further decrease in the count during the remainder of the infusion (PMID:37003030). During the first hour of infusion, median CLL cell membrane CD20 levels decreased to 65% of baseline and were 41% of baseline at the end of the infusion. Median serum rituximab concentration was 2 mcg/ml at 1h and 10 mcg/ml at the end of the infusion. Median complement (CH50) concentration decreased to 91% of baseline at 1 h and to 81% of baseline at the end of the infusion. The cessation of clearance of CLL cells from the circulation within 1h of starting rituximab therapy is thus unlikely to be caused by low rituximab levels, decreased CLL cell CD20 expression or low serum complement levels. We and others have previously shown that ADCP is the primary mechanism of clearance of anti-CD20 mAb opsonized B cells in both humans and mice. We hypothesized that clearance of circulating CLL cells decreases because residual circulating CLL cells are resistant to mAb induced ADCP. To test this hypothesis, we measured in vitro ADCP sensitivity of CLL cells sampled from patients before and after rituximab treatment.
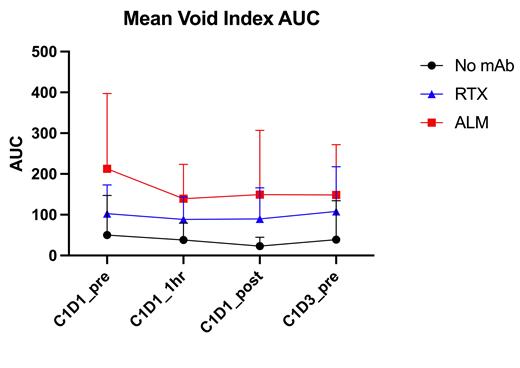
Methods: Blood specimens were collected from 13 patients prior to starting therapy (C1D1_pre), 1h after start of IV rituximab infusion (C1D1_1h), at end of rituximab infusion (C1D1_post), and 48 h after initiation of therapy (C1D3_pre). Sensitivity of circulating CLL cells to in vitro ADCP was measured using live cell time-lapse high-content microscopy imaging of phagocytosis of CLL cells by human monocyte derived macrophages (hMDM) as previously described (PMID:32005699). Phagocytosis was quantified using a void index and sensitivity of samples was compared using the area under the curve of the 2h void index plot (AUC). Experiments were done in triplicate with co-cultures of hMDM and CLL cells without mAb as controls. Rituximab (10 mcg/ml) was used to test CD20 mAb mediated ADCP and alemtuzumab (10 mcg/ml) to test non-CD20 mediated ADCP.
Results: Figure (median and 95% confidence intervals) shows that neither rituximab nor alemtuzumab induced ex-vivo ADCP as measured by median void index AUC was significantly altered by prior in vivo exposure of CLL cells to rituximab (Wilcoxon Match-Paired Sign test; all p>0.05).
Discussion: The rapid decrease in circulating CLL cells induced by IV rituximab occurred only within the first hour after initiation of mAb infusion. Our measurements show that serum rituximab and complement concentrations and CLL cell membrane CD20 levels should be adequate to support additional CLL cell clearance after 1h. We now demonstrate that residual circulating CLL cells remain sensitive to in vitro ADCP by both rituximab and alemtuzumab. This suggests that despite reduced levels of surface antigen, circulating cells remain sensitive to mAb mediated clearance. Alternative explanations for the limited clearance of circulating CLL cells include: 1. Saturation of the limited cytotoxic capacity of the innate immune effector cells (Kupffer cells and splenic macrophages) compatible with our previously reported data on finite macrophage ADCP capacity (PMID:32556153); 2. Mobilization of CLL cells from lymphoid tissue; and 3. Temporary sequestration of rituximab opsonized CLL cells in tissue (e.g. spleen or lungs), which is unlikely given the observed decrease in lymph node size following initiation of therapy. Ongoing research is testing these hypotheses.
Disclosures
Chu:Pfizer: Current holder of stock options in a privately-held company. Barr:AstraZeneca: Consultancy, Research Funding; Seattle Genetics: Consultancy; MEI Pharma: Consultancy; AbbVie: Consultancy; Genentech: Consultancy; Celgene: Consultancy; Morphosys: Consultancy; Gilead: Consultancy; Bristol Myers Squibb: Consultancy; Pharmacyclics LLC, an AbbVie Company: Consultancy; Janssen: Consultancy; TG therapeutics: Consultancy, Research Funding. Zent:AstraZeneca: Research Funding; GenMab: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal